O-glycan-specific Protein Digestion
OpeRATOR is an O-glycan-specific protease that digests proteins carrying core 1 O-glycans, N-terminally of the glycosylated (core 1) Ser and Thr residues.
Glycopeptides carrying O-glycans are generated, and the enzyme enables O-glycan profiling, O-glycopeptide mapping and site occupancy determination, as well as middle-level approaches using MS analysis.
- Reliable O-glycan-specific protease – digests N-terminally of Ser/Thr glycosylation sites
- Robust digestion for enhanced characterization of complex biopharmaceuticals
- High specificity - confident glycan site determination and mapping
OpeRATOR® Lyophilized
Lyophilized enzyme for digestion of mucin-type O-glycoproteins and peptidesAbout OpeRATOR®
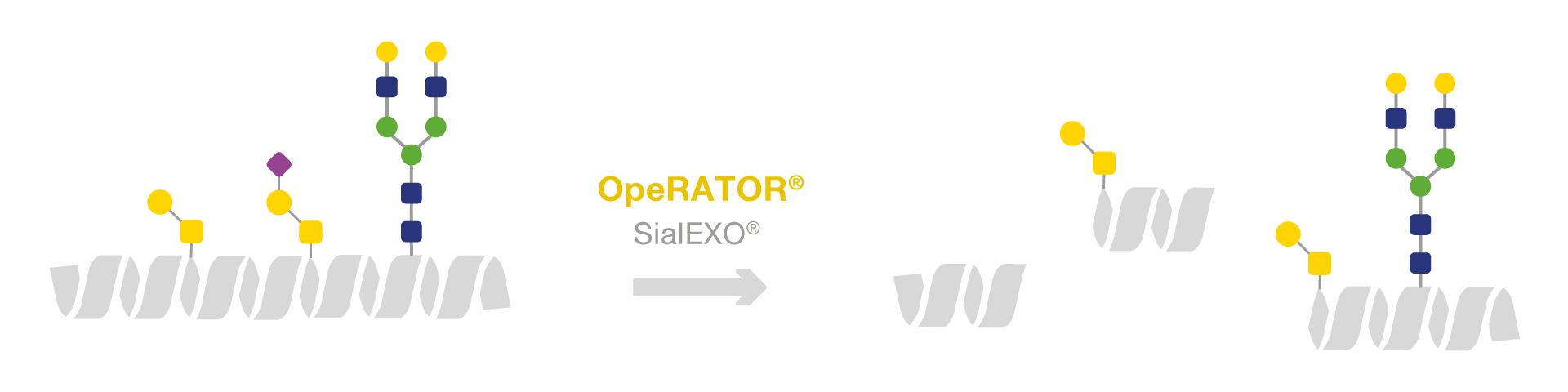

OpeRATOR is an endoprotease that catalyzes the hydrolysis of the peptide bonds of native mucin-type O-glycosylated proteins that are in direct proximity of the O-glycans. With high specificity, the endoprotease digests proteins at the N-terminus of O-glycans at serine or threonine residues.
O-glycans of mucin-type are required for OpeRATOR activity and the enzyme will not digest glycoproteins with only N-linked glycans. The enzyme is most active towards sites with asialylated core1 O-glycans. It also digests sialylated core 1 and core 3, but to a much lower extent. Removal of sialic acids is required for optimal performance, and therefore, SialEXO Lyophilized (sialidase mix) is included in the purchase.
OpeRATOR is derived from Akkermansia muciniphila and expressed in E. coli. The enzyme contains a His-tag and the molecular weight is 42 kDa. SialEXO is derived from Akkermansia muciniphila and expressed in E. coli. The enzymes in SialEXO Lyophilized also contain His-tags, and the molecular weights of these components are 43 kDa and 66 kDa, respectively.
OpeRATOR® Citations
OpeRATOR® Lyophilized
Lyophilized enzyme for digestion of mucin-type O-glycoproteins and peptides.
OpeRATOR Lyophilized is available as a lyophilized powder in 2000 unit vials for digestion of 2 mg O-glycosylated protein. Removal of sialic aids is required for optimal performance, and 2000 units if SialEXO Lyophilized is therefore included in the purchase.
- Flexible format for method development
- Possible to combine enzymes for improved efficiency
- Amenable to automated workflows
- Ready to use – just add water!
Available Products
For information on how to order, visit Place an Order or contact us directly at order@genovis.com.
Unit Definition
One unit digests ≥ 90% of 1 µg glycoprotein (TNFR) when incubated together with one unit of SialEXO in 20 mM Tris pH 6.8 at 37 °C for 2 h.
Content and Storage
OpeRATOR Lyophilized includes:
- 1 vial of OpeRATOR Lyophilized (G1-OP1-020) supplied lyophilized in TBS pH 7.6, with no preservatives added.
- 1 vial of SialEXO Lyophilized (G1-SM1-020) supplied lyophilized in TBS pH 7.6, with no preservatives added.
The product is shipped at ambient temperature, and should be stored at -20°C upon arrival. After reconstitution, the enzymes are stable for 1 month at +4-8 °C.
Instructions
Product Specification
Safety Data Sheet
Certificate of Analysis
FAQ and Support
Popular FAQ
OpeRATOR is a metalloprotease and as such highly sensitive to chelating agents such as EDTA. Concentrations >5mM lead to complete inhibition of the enzyme. OpeRATOR activity is also moderately inhibited by ZnCl2.
Yes, the enzymes work in combination and can be used to desialylate and digest an O-glycosylated protein.
The activity of OpeRATOR is significantly decreased in the presence of sialic acids. We recommend using SialEXO to remove the sialic acids. If this is not an option, then we suggest trying to digest both with and without SialEXO to evaluate the impact for your specific samples and requirements.
No, the enzyme recognizes mucin type glycosylation with an inner GalNAc linked to a hexose sugar.
The relative abundance and site occupancy of O-glycosylation may vary a lot in a glycoprotein sample, and this will result in the generation of peptides with variable sizes. In some cases, the peptides contain more than one O-glycan, i.e. OpeRATOR does not digest at every site in every molecule. However, the missed digestion sites differ from molecule to molecule, and the compiled data will provide valuable information on the O-glycosylated sites.