Automated antibody subunit analysis
Introduction
Middle-level analysis by LC-MS is a widely accepted analytical method for rapid characterization of mAbs and other IgG-based biopharmaceuticals. It can facilitate an understanding of multiple critical quality attributes (CQAs) such as glycosylation, oxidation, and C-terminal lysine clipping. Such detailed knowledge of post-translational modifications (PTM) is required for the development and manufacturing of biopharmaceuticals. FabRICATOR HPLC provides rapid on-column digestion of monoclonal antibodies with consistent subunit generation. The column contains immobilized FabRICATOR (IdeS) enzyme and digests IgG rapidly and specifically below the hinge, generating F(ab’)2 and Fc fragments without any risk of over-digestion, Figure. 1.
Figure 1. FabRICATOR digests IgGs to F(ab´)2 and Fc. After reduction, the size of the fragments Fd’, Fc/2 and LC are 23–25 kDa, which is well suited for high resolution MS analysis.


FabRICATOR HPLC Column 2D Instrument Setup
To reduce sample handling and provide an automated solution for sample preparation, FabRICATOR was covalently immobilized on a resin and packed in PEEK column hardware suitable for HPLC. Using the FabRICATOR HPLC column, antibody subunit generation can be automated using a standard HPLC-MS setup with only minor modifications. The resulting subunits generated by digestion on the FabRICATOR HPLC column are then trapped on the column head of a reversed-phase (RP) column connected in-line.
After uncoupling the enzyme column from the flow path using a column switching valve, the fragments can be reduced by injection of a reducing agent before being separated over the RP column and analyzed by mass spectrometry. This allows for a fully automated, completely hands-off workflow for analysis of mAb quality attributes (Fig. 2).
Robust Continuous On-column Digestion Performance
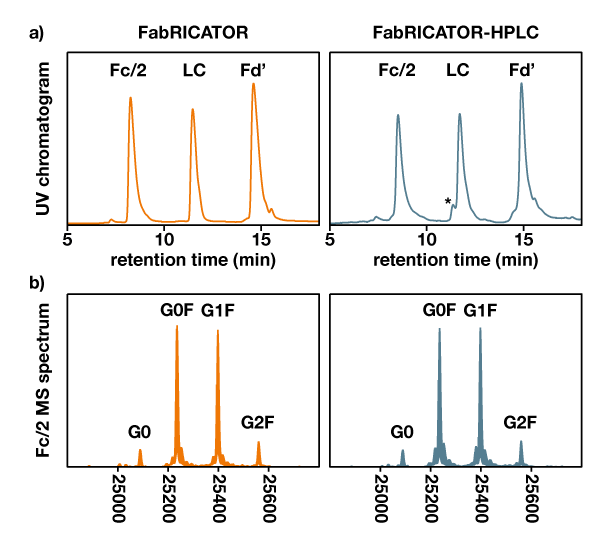
The enzymatic performance of the FabRICATOR column was compared to lyophilized FabRICATOR and analyzed using LC-MS. The yielded MS spectra are virtually indistinguishable from the ones obtained using the standard in-solution FabRICATOR protocol (Fig. 4).
A typical cell culturing process for expression of a monoclonal antibody takes between 10-14 days. To assess suitability of FabRICATOR HPLC for monitoring of mAb CQAs during this entire period, we tested the automated middle-level LC-MS approach for 14 days of continuous operation. A new trastuzumab sample (2 µg) was injected every 4 hours and digestion performance was measured by quantification of the intact HC as well as the Fc/2 and Fd’ fragments from the UV chromatogram.
Figure 4. Comparison between digestion on FabRICATOR HPLC and FabRICATOR in solution. a) UV chromatogram of the trastuzumab subunits generated by the in-solution FabRICATOR protocol (left, orange) and the automated FabRICATOR HPLC workflow (right, teal). The asterisk marks LC fragments that are not completely reduced with one intramolecular disulfide bridge intact. b) Deconvoluted MS spectra of the Fc/2 fragment generated by in-solution FabRICATOR digestion (left, orange) or the automated FabRICATOR HPLC workflow (right, teal)
In excess of 95% of the sample was digested during the entire 14-day period (Fig. 5a). We also analyzed Fc glycosylation by middle-level LC-MS and compared it to results obtained from a standard in-solution FabRICATOR workflow (5). The Fc glycosylation profiles we obtained using the automated FabRICATOR HPLC workflow were stable during the entire 14-day experiment with standard deviations of <0.5% for all glycoforms. The results also corresponded well to a data set produced using the in-solution FabRICATOR protocol (Fig. 5b) as well as previously published data on trastuzumab glycosylation (3).
Figure 5: FabRICATOR HPLC performance during a 14-day period of continuous operation. a) Digestion efficiency of FabRICATOR HPLC was followed for 14 days with 6 trastuzumab samples (2 µg each) being analyzed automatically per day. The digestion percentage was determined from the obtained UV chromatograms. b) Comparison of the obtained Fc glycosylation profiles from middle-level LC-MS analysis using the standard in-solution FabRICATOR protocol (n=10, orange) or the automated FabRICATOR HPLC workflow (n=28, 2 samples per day, teal). The bars represent the mean, the error bars the standard deviation.
Monitoring Ongoing Reactions
The change in quality attributes of a mAb over the course of a cell culturing process is highly dependent on process parameters. While FabRICATOR HPLC can be used in a standard LC-MS setup for routine analysis, more advanced configurations are possible, for example with a 2D-LC. Ultimately a bioreactor can be connected directly to the MS in an automated online middle-level workflow, Figure. 3. This can significantly reduce operator time, sample handling errors and increase the throughput.
Figure 3. Possible FabRICATOR HPLC workflow. b) Potential set-up for an online monitoring workflow of a bioreactor using FabRICATOR HPLC. Samples are taken from the culture supernatant at desired intervals. After potential sample preparation, IgGs are digested into subunits and separated using a 2D-HPLC system followed by analysis of critical quality attributes by mass spectrometry