NEW! We’re Launching IgASAP™ for Digestion of IgA!
IgASAP Sub1 is an IgA1-specific protease that digests human IgA1 at one specific site above the hinge, generating intact and homogeneous Fab and Fc fragments.
The enzyme enables generation of intact monovalent Fab fragments as well as middle-level analysis of IgA1, which facilitates IgA1 characterization during, for example, the development of vaccines, therapeutics, and diagnostics.
- Enables middle-level characterization and quality control of IgA1
- Fast – generates homogeneous Fab and Fc fragments within 1 hour
- Highly specific – one digestion site above the hinge of human IgA1
Site-specific Digestion of Human IgA1
To demonstrate the site-specificity of IgASAP Sub1, a commercially available human myeloma IgA1 was digested with IgASAP Sub1 Lyophilized for 1 hour at 37°C and compared to undigested IgA1. As IgA1 carries multiple N- and O- glycans, the samples were treated with OmniGLYZOR Kit, including the additional deglycosylation step under denaturing conditions using PNGaseF Lyophilized to remove all N-glycans, to facilitate the data interpretation.
The digestion using IgASAP Sub1 is fast, easy, and carried out under physiological conditions, and the specificity of the enzyme prevents overdigestion of IgA1. Despite the heterogenous starting material, the data clearly shows the single digestion site in the upper hinge region of human IgA1 (VPSTPP / TPSPST) and emphasizes the advantages of using a middle-level workflow to generate high resolution mass data for such complex samples.
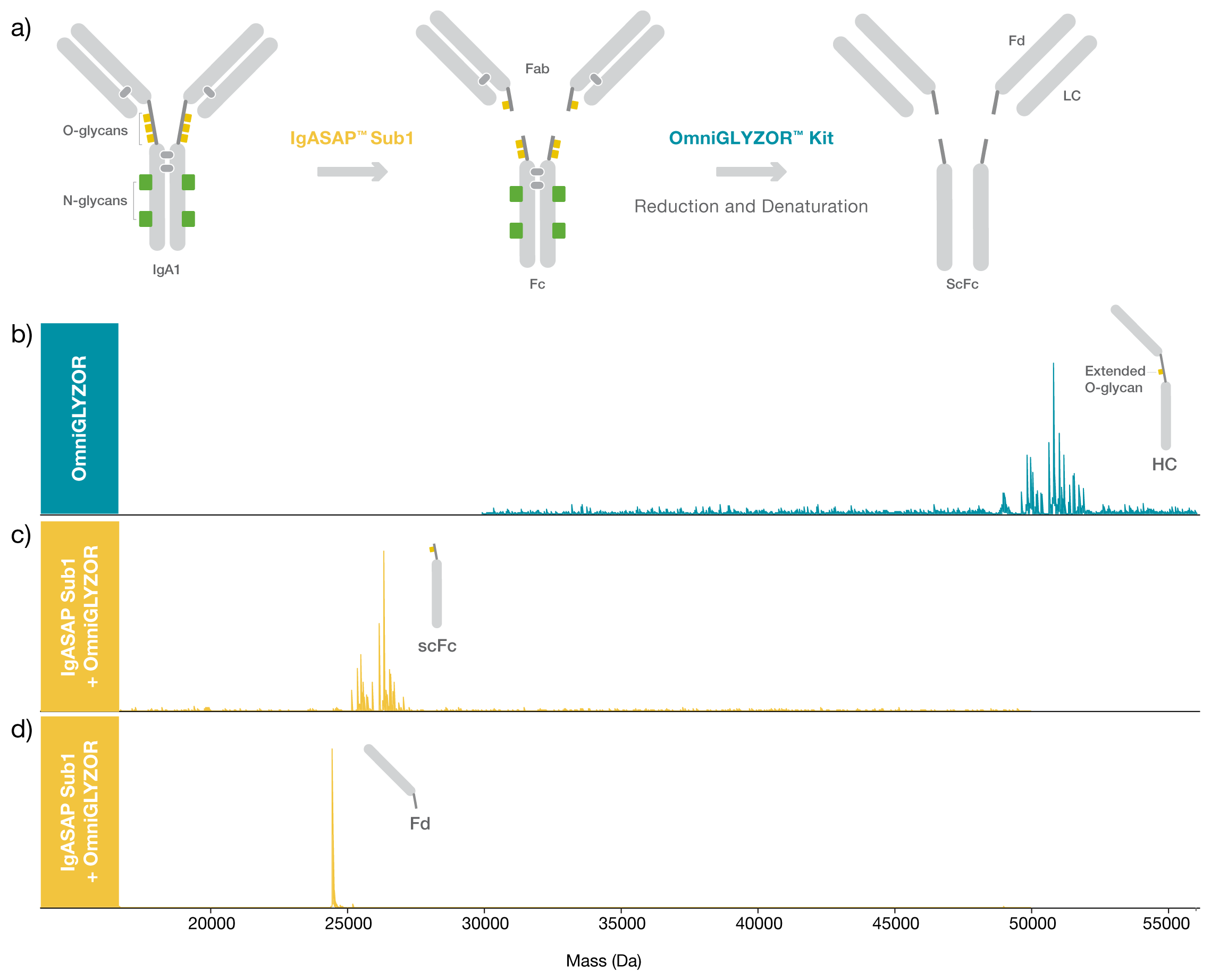
Figure 1. Site-specific digestion of IgA1. a) Schematic illustration of the workflow. Deconvoluted mass spectra of N- and O-deglycosylated b) heavy chain (HC) of a commercially available IgA1 from human myeloma and N- and O-deglycosylated c) scFc fragment and d) Fd fragment after digestion using IgASAP Sub1 for 1 hour at 37°C. The heterogeneity of the HC and scFc fragments derives from several C-terminal truncations and a remaining extended O-glycan, not removed by OmniGLYZOR. The samples were separated by reversed-phase chromatography (ACQUITY Premier Protein BEH C4, 300 Å, 1.7 µm 2.1 x 100 mm, Waters™) and analyzed with ESI-QTOF MS (Bruker Impact II).
IgASAP Sub1 Lyophilized – Above hinge digestion of 1 mg IgA1.