F(ab')2 and Fc/2 Fragments
Antibody Fragmentation using FabRICATOR
Antibody fragments such as F(ab’)2 are commonly used as tools in research and spans a broad range of applications. To generate these fragments, enzymatic trimming of an intact antibody is a popular route. In this section, generation of F(ab’)2 and Fc fragments by utilizing the highly specific enzyme FabRICATOR (IdeS) is discussed.


Figure 1. Digestion of human IgG1 by FabRICATOR generates discrete F(ab')2 and Fc fragments.


Enzymatic generation of F(ab’)2 and Fc/2 fragments
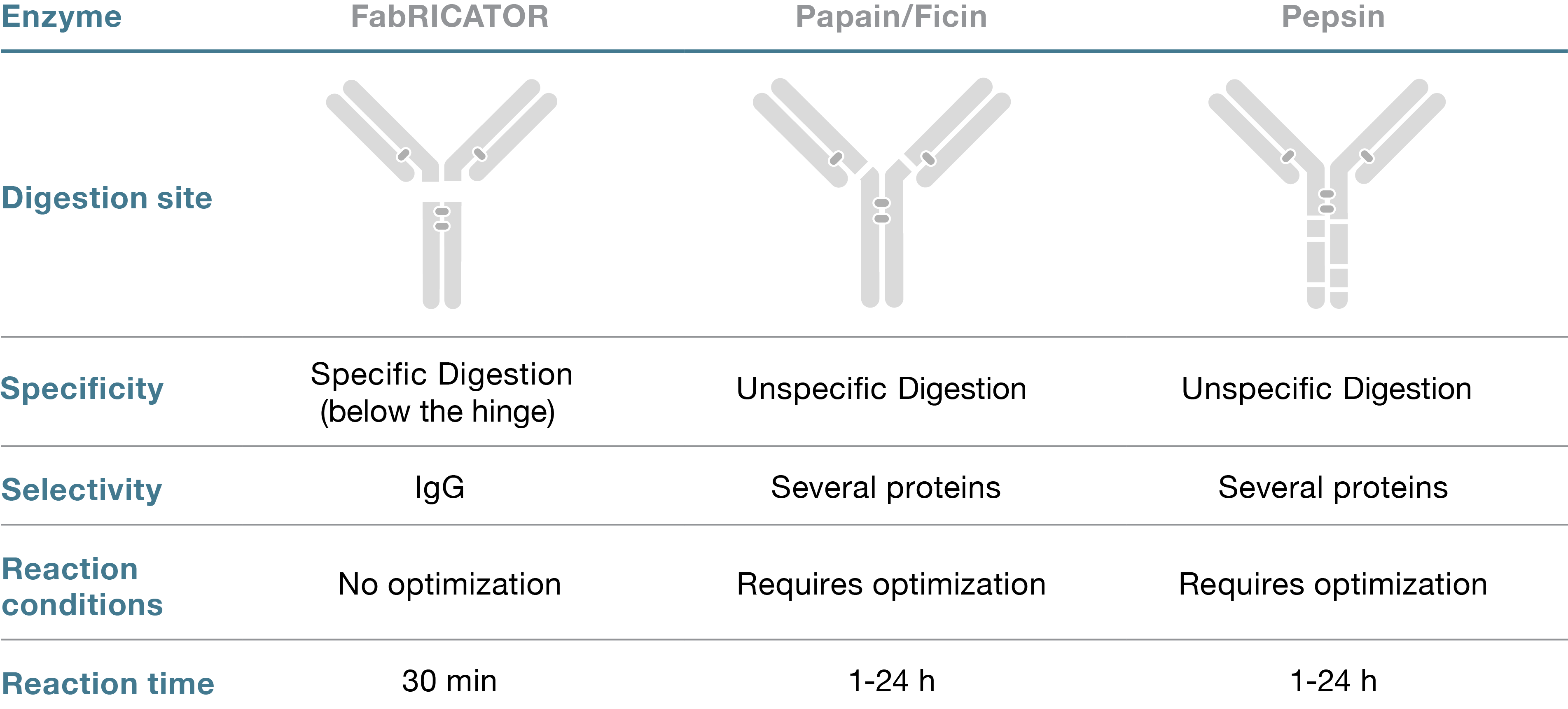
Homogeneous F(ab´)2 and Fc/2 fragments can be generated by digestion at a single site below the hinge of IgG using the site-specific cysteine protease FabRICATOR (IdeS). The enzyme is specific for the many subclasses of the IgG antibody isotype and no other substrates have been reported. The specificity of FabRICATOR allows for fast, easy and robust digestion at neutral pH to generate homogenous fragments with preserved immunoreactivity (Fig. 1). In addition, there is no risk for further degradation or over-digestion, a challenge otherwise commonly associated with other proteolytic enzymes like pepsin, papain and ficin (Table 1).
FabRICATOR can be used on all human subclasses of IgG as well as humanized and chimeric IgG. The enzyme is also active on IgG from other species including some classes of IgG from rat, monkey, rabbit and sheep. The high specificity of FabRICATOR can in some cases therefore render it sensitive to mutations in the hinge region and compromise the enzymatic activity. Fragments from mouse IgG2a and IgG3 can be generated by FabRICATOR Z that has an improved efficiency compared to FabRICATOR for digestion of these IgGs. Mouse IgG1 however, has a different sequence below the hinge and is not digested by FabRICATOR Z. For this antibody species FabULOUS and FabULOUS Fab kit can be used to create Fab fragments.
Pure F(ab')2 and Fc fragments with FabRICATOR Immobilized (formerly FragIT)
Some application such as binding studies are highly sensitive and therefore require pure antibody fragments without residual enzyme in the final preparation. Our IgG specific proteases are therefore also available in immobilized formats for fast and easy generation of homogenous F(ab’)2 and Fc/2 fragments.
The FabRICATOR Immobilized contains immobilized FabRICATOR (Ides) to prepare F(ab’)2 and Fc fragments from several species of IgG (for rabbit IgG, please inquire), while mouse IgG2a and IgG3 can be processed using the FabRICATOR Z Fab2 Kit containing FabRICATOR Z Immobilized. To generate and purify intact F(ab’)2 and Fc/2 fragments, trastuzumab was processed using the FabRICATOR Fab2 Kit and analyzed by SDS-page (Fig. 2). First, the antibody was digested on the FabRICATOR Immobilized spin column at RT for 15 minutes followed by a short centrifugation step. The F(ab’)2 fragments were purified on the CaptureSelect ™ spin column and the bound Fc fragments eluted in a single step by lowering the pH followed by immediate adjustment back to neutral pH. The procedure from digestion to collection of fragments using FabRICATOR Fab2 Kit takes about one hour.
Figure 2. Separation of antibody fragments on non-reducing SDS-PAGE. Lane 1) kDa markers, Lane 2) intact intact antibody, Lane 3) Fab'2 and Fc fragment after FabRICATOR Immobilized digestion, Lane 4) F(ab')2 in flow-through over Fc affinity capture column, Lane 5) Eluted Fc fragment


Fab’ fragments from F(ab’)2
The F(ab’)2 produced by digestion with FabRICATOR Lyophilized or FabRICATOR Immobilized resin can easily be separated to generate two sulfhydryl containing Fab’ fragments. A single reduction step with a mild reducing agent as 2-mercaptoethylamine (2-MEA) or cysteamine yields the Fab’ fragments in a simple 2-hour procedure. The generated Fab’ fragments contain the hinge thiols available for conjugation through standard maleimide chemistry and there is no need to remove the Fc fragments before conjugation. If the preparation of F(ab’)2 is performed using the FabRICATOR Fab2 Kit, the solution contains pure Fab’ fragments. The SDS-PAGE analysis shows the purity of the fragments generated from trastuzumab using of the FabRICATOR Fab2 Kit followed by mild reduction with 2-MEA (Fig. 3).
Protocol procedure applied on a human IgG1 with reduction procedure adapted from Bioconjugate Techniques by Hermanson. The reduction is done in presence of EDTA to prevent re-oxidation of the sulfhydryls by metal catalysis.
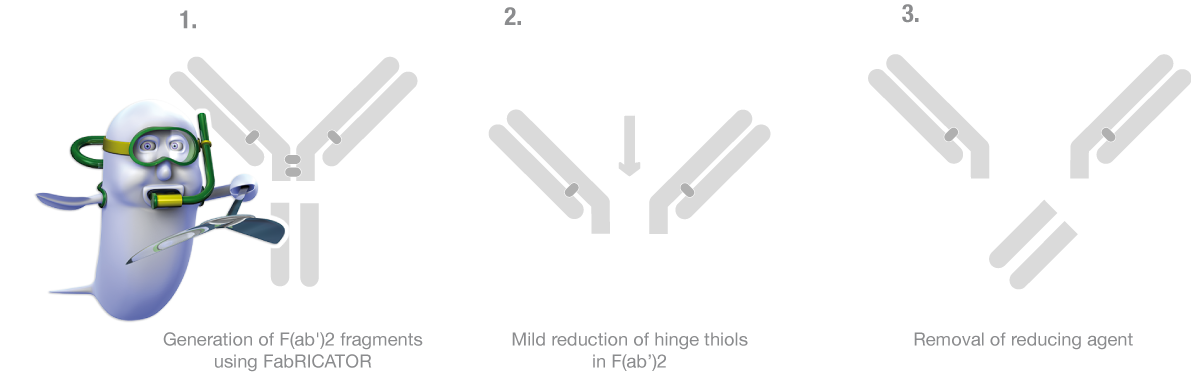
1 - Generation of F(ab')2 fragments using FabRICATOR
FabRICATOR Lyophilized and FabRICATOR Immobilized digest below the hinge region in 30 minutes, generating a homogenous pool of F(ab')2 fragments and Fc fragments. Reaction should be performed in a buffer containing EDTA, for example, 50 mM Phosphate, 150 mM NaCl, 5 mM EDTA, pH 7
2 - Mild reduction of hinge thiols in F(ab’)2
Prepare a stock solution of 500 mM 2-MEA in 50 mM Phosphate, 150 mM NaCl, 5 mM EDTA, pH 7.2. Next, add 2-MEA from the stock to the digested IgG to a final concentration of 50 mM 2-MEA and incubate the solution for 37°C for 90 minutes. There is no need to remove the FabRICATOR enzyme if present.
3 - Removal of reducing agent
The solution contains Fab’ fragments, Fc fragments, 2-MEA, and potentially FabRICATOR enzyme if FabRICATOR Immobilized was not used. To remove the 2-MEA a desalting column with a suitable size to process the sample volume is used for rapid and convenient processing.